
The adoption of eCTD 4.0 represents a crucial step toward modernizing regulatory submissions worldwide. With benefits like enhanced document tracking and metadata-driven efficiencies, it’s no surprise that global agencies are embracing this shift.
But where does EMA stand? Unlike the FDA, which has outlined clear implementation phases, EMA is taking a more measured approach, starting with pilot programs and technical evaluations. In this blog, we break down EMA’s plans for eCTD 4.0, its potential impact on regulatory filings, and how your company can stay ahead.
More importantly, has EMA mandated eCTD 4.0 yet? Let’s dive in.
What is eCTD 4.0 and how is it different from eCTD 3.2.2?
The Electronic Common Technical Document (eCTD) is the internationally accepted format for regulatory submissions. It standardizes the way pharmaceutical companies submit drug applications and manage lifecycle updates across different markets.
The current version, eCTD 3.2.2, has been in use for years. However, regulatory bodies, including EMA, have been working toward eCTD 4.0, which is based on the Health Level 7 (HL7) Regulated Product Submission (RPS) standard. This update enhances submission efficiency and regulatory lifecycle management.
Key differences between eCTD 3.2.2 and eCTD 4.0:
- Better Lifecycle Management: Improved tracking of document versions, reducing duplication.
- More Flexible Granularity: Allows structured document reuse across different submissions.
- Enhanced Metadata Handling: More structured metadata, making regulatory interactions smoother.
- Greater Interoperability: Designed to work better with evolving digital regulatory systems globally.
- While these changes offer numerous benefits, the transition to eCTD 4.0 requires preparation, investment, and an understanding of regulatory expectations.
EMA’s Stance on eCTD 4.0 Adoption
The big question on everyone’s mind is: Has EMA mandated eCTD 4.0? The answer is not yet, but the transition is in progress.
Current Status of eCTD 4.0 in the EU:
- As of now, eCTD 3.2.2 remains the mandatory standard for submissions in the EU.
- EMA has been actively evaluating eCTD 4.0 but has not officially mandated its use.
- The agency is working alongside other regulatory bodies, such as the FDA and Japan’s PMDA, to align global standards.
- No official transition deadlines have been set, but EMA has indicated that eCTD 4.0 will eventually become mandatory.
EMA's Approach: Phased Rollout with a Pilot Program
Unlike the USFDA, which has outlined a phased implementation plan, EMA is taking a structured approach. The agency has launched a Technical Pilot Program to test eCTD 4.0 implementation with industry stakeholders.
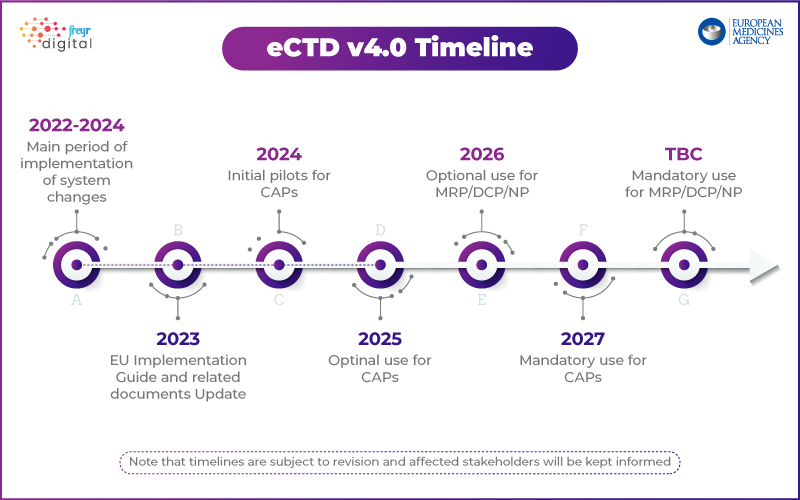
Key highlights from EMA’s eCTD 4.0 strategy:
- Step 1: Focus on technical interoperability with regulatory tools.
- Future Phases: Mock submissions, lifecycle management, and grouped submissions.
- Draft implementation package available with controlled vocabularies and validation criteria.
EMA is committed to ensuring a smooth transition, and life sciences companies should follow updates on the official EMA eCTD page.
Challenges & Considerations for Life Sciences Companies
While eCTD 4.0 offers clear benefits, adopting it is not without challenges. Here are some key considerations for pharmaceutical and biotech companies:
- Regulatory Complexities
- EMA's gradual transition means companies must be prepared for dual submission processes (eCTD 3.2.2 and eCTD 4.0).
- Different regions will have different adoption timelines, requiring companies to stay agile.
- Technical Challenges
- Data migration from eCTD 3.2.2 to 4.0 requires careful planning.
- Validation rules will change significantly, demanding updated software and tools.
- Companies need to invest in automation and AI-driven solutions to ensure compliance.
- Compliance Risks
- Incorrect metadata mapping or failure to comply with new document granularity requirements could lead to submission rejection.
- Regulatory teams need proper training to understand new validation rules.
- Impact on Existing Workflows
- Companies will need to adapt internal processes to accommodate eCTD 4.0.
- Increased collaboration between regulatory, IT, and submission teams will be necessary.
The key takeaway? Companies need to start preparing now, even though EMA has not yet mandated eCTD 4.0.
How Companies Can Prepare for EMA’s eCTD 4.0 Transition
Even though EMA hasn’t set an official deadline, proactive companies will benefit from early preparation. Here’s how:
- Assess Current Submission Processes
- Identify gaps in existing eCTD 3.2.2 workflows.
- Evaluate how new lifecycle management rules will impact ongoing submissions.
- Invest in the Right eCTD 4.0 Tools
- Select regulatory submission software that is eCTD 4.0-compliant.
- Ensure software supports HL7 RPS standards and metadata automation.
- Train Regulatory & IT Teams
- Conduct workshops and training sessions on eCTD 4.0 changes.
- Provide guidelines for metadata tagging and lifecycle tracking.
- Engage with EMA and Industry Peers
- Participate in EMA’s pilot programs and regulatory consultations.
- Stay updated on EMA's latest guidance and regulatory updates.
- Conduct Testing & Validation
- Run internal test submissions using eCTD 4.0 structures.
- Identify potential issues in document granularity and metadata mapping before EMA’s final mandate.
By starting now, companies can ensure a smooth transition without disruptions to regulatory filings.
What’s Next? Future of eCTD 4.0 in Europe
While the full transition timeline remains unclear, EMA's long-term strategy involves:
- Standardizing submissions across global markets, improving consistency and efficiency.
- Reducing approval timelines through better submission lifecycle tracking.
- Integrating AI and automation into regulatory workflows to enhance compliance.
In the next few years, we can expect more clarity from EMA, including official transition timelines and new validation criteria.
Conclusion
While EMA has not yet mandated eCTD 4.0, it is only a matter of time before it becomes the standard for regulatory submissions in the EU. Companies that proactively prepare will gain a competitive advantage, ensuring compliance and avoiding last-minute regulatory hurdles.
Key Takeaways
- eCTD 3.2.2 remains the current standard, but eCTD 4.0 is on the horizon.
- EMA is taking a gradual approach, with no official mandate yet.
- Companies should start preparing now to ensure a smooth transition.
- Investing in new technology, training teams, and testing submissions will be key.
Call to Action
Stay updated with EMA’s regulatory announcements and start assessing your readiness for eCTD 4.0. The sooner you prepare, the easier the transition will be when the mandate arrives.
Would you like assistance in choosing eCTD 4.0-compliant software or understanding how this transition affects your business? Feel free to reach out to us.
