A huge thanks to Freyr Digital for the excellent support in turning around 100 (approx..) lit references, which were needed in a short period in support of a New Drug Application (NDA) submission. Really appreciate their dedication and hard work in making this possible. This is a critical submission for us and therefore I wanted to take the time to let you know that the submission team recognize your contribution and are extremely grateful for your support not only for these but also for the hundreds of reports, which you’ve republished to make submission-ready for this major submission.
- Switzerland based Global Pharma company
accelerate and streamline
Streamline Your Regulatory Journey with Unified RIMS Solution:
With Freyr SPAR, streamline your registrations for seamless HA approvals.
Navigate multiple products and markets effortlessly under a unified platform.
Stay ahead of the curve and maintain compliance with dynamic global Regulations.
One-click access to unified information, no matter where you are in the world.
Manage, organize, and interact with all Regulatory documents with precision.

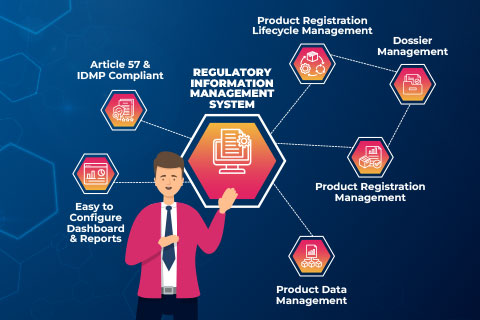
KEY FEATURES AND FUNCTIONALITY
Seamlessly Integrate, Track, and Optimize Regulatory Information Management.
Experience end-to-end registration management throughout the entire product lifecycle, from variations to obligations and commitments, ensuring consistency and efficiency.
Freyr SPAR is primed for IDMP standards and offers seamless integration with platforms such as eDMS, eCTD publishing, submissions, Label change tracking, XEVMPD, and SPL-SPM.
Utilize our intelligent, wizard-driven approach for record creation across multiple products and enhance your search capabilities with powerful filters to pinpoint specific records.
Centralize all your Regulatory documents in one repository and manage crucial metadata with just a few easy clicks.
Deliver insights tailored to your needs with highly customizable reports and dashboards.
Access and navigate Freyr SPAR from anywhere through its intuitive web browser interface, ensuring flexibility, interactivity, and user-friendliness.
REGULATORY INFORMATION & DOCUMENTATION MANAGEMENT
Excellence Embedded: Set It Up and Let Freyr SPAR Do the Magic
Ensure your product data remains consistently in sync with the ever-evolving global regulations. With automated validation systems, SPAR aligns with international standards effortlessly.
Harness the power of integrated SPOR within our system. With the inclusion of reference data, Freyr SPAR offers tailored customization to suit your specific data migration needs, ensuring seamless transitions and adaptability.
From clinical trials, product registration, to post-market surveillance, monitor and manage every pivotal step with precision.