
The world of pharmaceutical regulations is about to get a major upgrade with the implementation of eCTD 4.0. This standardized format promises a smoother journey for new drug applications submitted to regulatory bodies. But for companies eyeing a slice of the $141.5 billion Japanese pharmaceutical market, there's a crucial twist – Japan is accelerating the adoption timeline!
Below is an analysis of these highlights:
- Mandatory Adoption: Contrary to those in other areas that have voluntary phases before mandatory adoption, by 2026 Japan aims to make use of eCTD 4.0 mandatory.
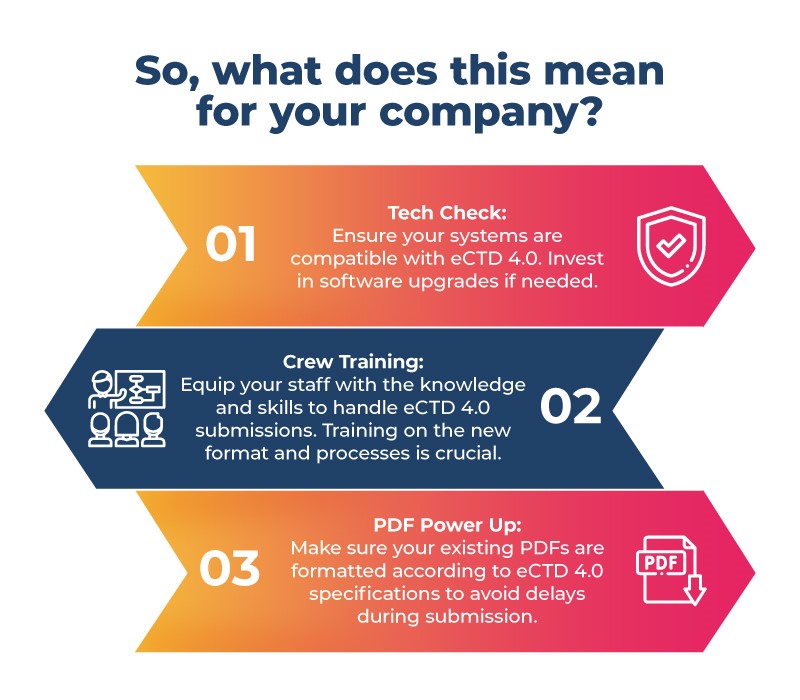
- Preparation is Key: Preparation is key due to time constraints and absence of a direct migration path from Japan PMDA format JP1.0 to eCTD 4.0, focusing on a better system will assist pharmaceutical companies in Japan during the transition process. These steps include updating software, training staff and preparing PDFs for the new format
- Resources Available: The Pharmaceuticals and Medical Devices Agency (PMDA) of Japan has published resources which can guide companies through this change such as “ICH Electronic Common Technical Document eCTD 4.0 Implementation Guide” with explanations on changes made and best practices available.
Here are some reasons why you shouldn’t miss out on this numbers game:
- Fast-Track to eCTD 4.0: Unlike other regions where the process is phased, Japan is targeting a compulsory adoption of eCTD 4.0 as early as in 2026. That is three years before the deadline set by US FDA for 2029! With such an aggressive timeline, it means that Japan has taken the lead in terms of global eCTD incorporation.
- No Room for Error: JP could not be directly converted to eCTD 4.0 unlike its previous versions. This means that pharmaceutical firms have to be extra ready for this so as to prevent any delays in submission which may cost them millions in revenue.
- Helping Hand For A Smooth Landing: The Pharmaceuticals and Medical Devices Agency (PMDA) of Japan understands the challenges. They have come up with resources like implementation guides designed for guiding companies through these modifications with ease and landing well in Japanese market. (https://www.pmda.go.jp/english/).
If you're developing drugs for the massive Japanese market, it's time to buckle down! Here's your pre-flight checklist:
The transition to eCTD 4.0 promises increased efficiency and global standardization throughout the drug approval process of pharmaceuticals. Your company can anticipate this move in Japan for a smooth transition and partake of the countless opportunities afforded by this new era. Remember that failing to prepare is preparing to fail- so, be ready to soar with eCTD 4.0 in Nippon.
The benefits and chances acquired are attractive for the pharmaceutical industry. However, it does have its own share of difficulties along the way which must be sorted out. In order to navigate seamlessly through an ever-changing regulatory environment, companies may seek partnerships with reliable consultants such as Freyr. Embrace the future of regulatory submissions in Japan with our unparalleled eCTD software, Freyr SUBMIT PRO, which is 21 CFR Part 11 standards compliant and has a flexible deployment model. Contact us today to learn more and embark on a journey towards enhanced efficiency and success. Request a demo today.
