
In regulatory submissions, the difference between a flawless, on-time submission and one riddled with delays often comes down to one key factor: communication. When teams aren’t aligned, and information is scattered, even the most well-planned submissions can become chaotic. But what if you could centralize communication and data exchange to avoid these pitfalls entirely?
Managing eCTD submissions is a complex task which makes collaboration among internal teams and external partners integral to achieving a successful submission. With so many moving parts, communication breakdowns, version control issues, and data inconsistencies can easily cause critical delays and errors. Even the most skilled teams face challenges juggling feedback, managing revisions, and ensuring secure data sharing. In this blog, we’ll discuss the most common challenges in communication & data exchange and discuss how using the right technology to streamline Your communication can make or break your eCTD submissions.
The Communication and Data Exchange Challenge
Effective eCTD submissions hinge on the smooth exchange of information between different teams and partners. However, this can be easier said than done:
- Scattered Communication Channels: When teams rely on a mix of emails, chat tools, and sporadic meetings, information gets siloed. Important updates can be overlooked, leading to gaps in the submission process.
- Data Inconsistencies and Version Control Issues: Multiple contributors working on the same documents often lead to version mismatches and conflicting data. Without clear control, teams may inadvertently submit outdated or inaccurate information.
- Delayed Feedback Loops: Regulatory submissions require timely feedback from both internal and external stakeholders. But with no centralized platform, gathering and incorporating input can take longer than expected, creating bottlenecks.
- Security Risks in Data Exchange: Handling sensitive regulatory data across multiple platforms introduces vulnerabilities. Ensuring that data is shared securely and remains accessible only to authorized users is critical.
Tips and Tricks for Enhancing Collaboration in eCTD Workflows
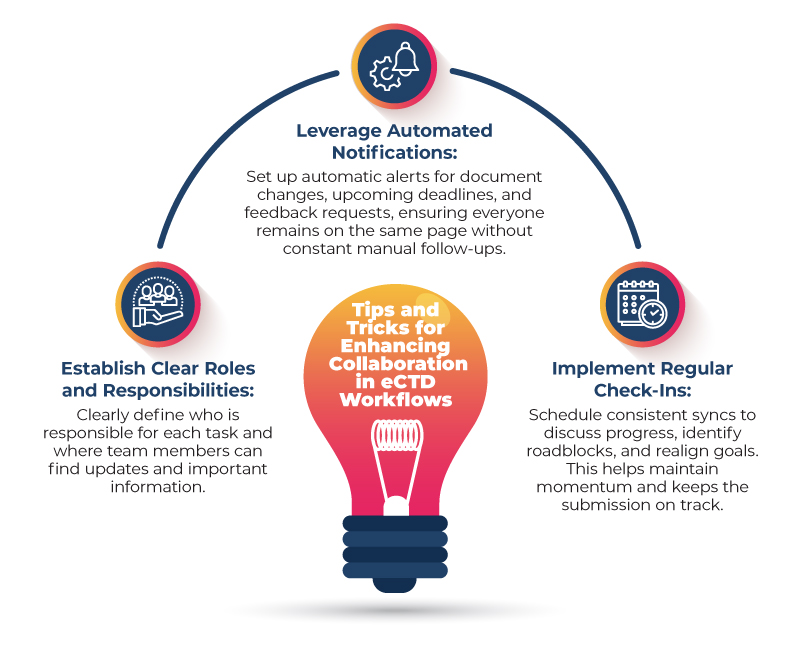
How Freyr SUBMIT PRO Simplifies Communication and Collaboration
Effective collaboration is crucial for successful eCTD submissions. Regulatory software offers centralized platforms where teams can collaborate in real-time, share documents, and communicate seamlessly. This can improve overall team productivity by up to 40%1.
Freyr SUBMIT PRO is designed to address these challenges by providing a unified, integrated platform for managing every aspect of your eCTD submission:
- Effective Communication: By consolidating all discussions, updates, and document revisions in one platform, Freyr SUBMIT PRO eliminates the confusion of scattered communication channels. Team members and external partners can easily collaborate in real-time, ensuring that everyone stays aligned throughout the submission process.
- Secure Data Sharing: With role-based access and built-in version control, Freyr SUBMIT PRO ensures that only authorized users can view and edit documents. This not only improves data integrity but also reduces security risks associated with multiple platforms.
- Seamless Integration: Seamless synchronization with your legacy systems or electronic document management systems guarantees a smooth workflow transition.
- CentralizedPlatform: Using regulatory software that offers centralized platforms can significantly enhance collaboration. These platforms allow teams to work together in real-time, share documents, and communicate seamlessly.
In addition to streamlined communication, Freyr SUBMIT PRO includes essential tools like submission tracking, an eCTD validator, and a query management system to make the entire process more efficient and less stressful.
Making eCTD Submissions Seamless
In regulatory submissions, communication and data exchange can make or break the entire process. With the right tools, like Freyr SUBMIT PRO, you can centralize communication, ensure data consistency, and reduce the risk of errors—all while meeting your regulatory deadlines with confidence.
Ready to transform your eCTD workflows? Discover how Freyr SUBMIT PRO can simplify your submission process and enhance collaboration across your teams. Contact us.
