
Electronic Common Technical Document (eCTD) submissions are a cornerstone in the life sciences industry, enabling streamlined and efficient regulatory processes. This is the reason companies globally have mandated the use of eCTD. However, the creation and submission of eCTDs come with their own set of challenges. In this comprehensive guide, we will explore these challenges and how regulatory software can help overcome them, ensuring compliance and maximizing return on investment (ROI).
The Electronic Common Technical Document (eCTD) has emerged as the global gold standard for drug regulatory submissions. The first version of eCTD was finalized in 2003, and while it has provided significant productivity benefits to both health authorities and the industry, additional improvements are needed to address evolving requirements in several key topic areas. This latest version eCTD v4.0 offers remarkable benefits such as increased efficiency and precision, but it also brings unique challenges for countries transitioning to this advanced format.
This blog will take you through the following pointers applicable for your Regulatory compliance needs.
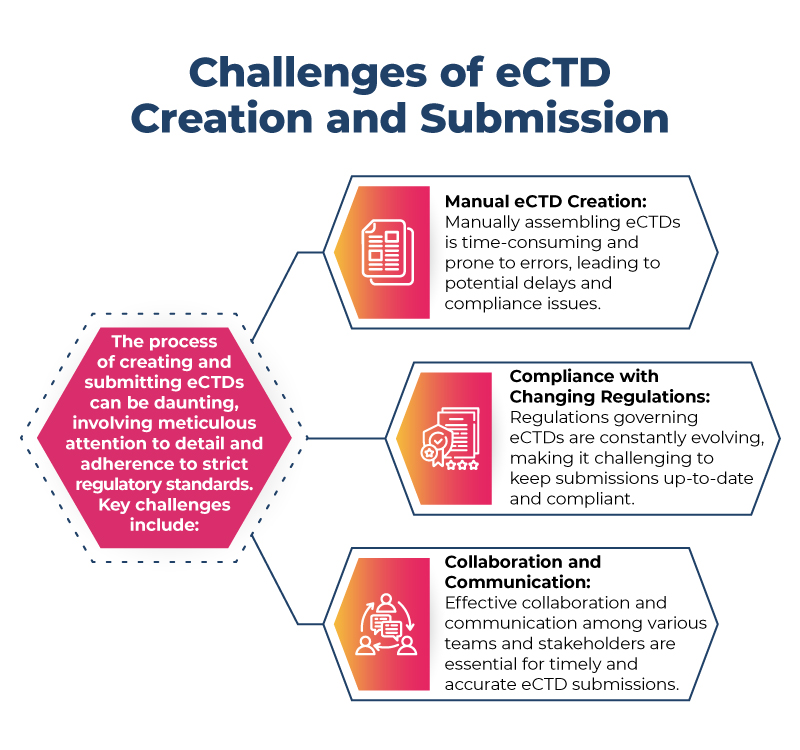
Challenges of eCTD Creation and Submission
Choosing the Right eCTD Software for Your Regulatory Needs
Best Practices for eCTD Management with Regulatory Software
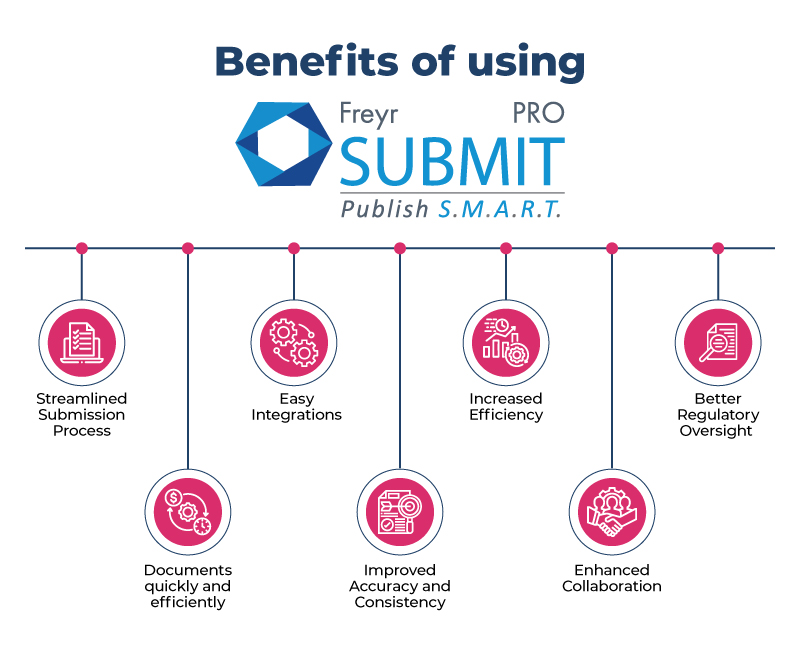
Benefits of using Freyr Submit PRO
Overview of eCTD’s
Let us dive into the world of eCTD’s and know more about it.
- Overcoming the Burden of Manual eCTD Creation
Manual eCTD creation is labor-intensive and increases the risk of errors. Regulatory software can automate many aspects of eCTD assembly, reducing the burden on regulatory teams. Automation ensures that documents are correctly formatted and organized, minimizing the risk of submission rejection due to technical errors.
- Ensuring eCTD Compliance with Ever-Changing Regulations
Regulatory software is designed to keep pace with the latest regulatory requirements. It can automatically update compliance standards and provide guidance on adhering to current regulations. This ensures that your eCTD submissions are always in line with the latest guidelines, reducing the risk of non-compliance and potential delays.
- Streamlining Collaboration and Communication in eCTD Workflows
Effective collaboration is crucial for successful eCTD submissions. Regulatory software offers centralized platforms where teams can collaborate in real-time, share documents, and communicate seamlessly. This streamlines the entire workflow, from document creation to final submission, ensuring that all stakeholders are on the same page and reducing the likelihood of miscommunication.
- Maximizing ROI with Regulatory Software for eCTDs
Investing in regulatory software for eCTD submissions can yield significant ROI. Automation reduces the time and resources required for manual processes, allowing regulatory teams to focus on higher-value tasks. Additionally, the reduction in errors and rejections leads to faster approvals and quicker time-to-market for new products, further enhancing ROI.
- Future-Proofing eCTD Processes with AI Innovation
Artificial intelligence (AI) is playing an increasingly important role in regulatory affairs. AI-powered regulatory software can analyze vast amounts of data, identify patterns, and predict potential issues before they arise. This proactive approach helps future-proof eCTD processes, ensuring that submissions are not only compliant but also optimized for efficiency.
Choosing the Right eCTD Software for Your Regulatory Needs
Selecting the right eCTD software is crucial for the success of your regulatory submissions. Factors to consider include:
- Ease of Use: The software should be user-friendly and intuitive, reducing the learning curve for your team.
- Compliance Features: Ensure that the software stays updated with the latest regulatory requirements and provides comprehensive compliance support.
- Collaboration Tools: Look for software that facilitates easy collaboration and communication among team members.
- Scalability: Choose software that can grow with your organization and handle increasing volumes of submissions.
Best Practices for eCTD Management with Regulatory Software
To maximize the benefits of regulatory software for eCTD submissions, consider the following best practices:
- Regular Training: Keep your team trained on the latest features and updates of the software.
- Standardized Processes: Establish standardized processes for eCTD creation and submission to ensure consistency and efficiency.
- Continuous Improvement: Regularly review and optimize your eCTD workflows to identify areas for improvement and enhance overall performance.
To stay ahead in this competitive industry, it is imperative for pharma companies to embrace regulatory tech products and harness the potential they offer.
By embracing Freyr Submit PRO you can harness the true potential of regulatory submissions to unparalleled heights.
Transitioning to eCTD might seem like a daunting task, but fear not! With the right strategy and guidance, countries can conquer this challenge and unlock the full potential of eCTD wonders. Partnering with vendors like Freyr, who have a reach in about 20 countries around the world and are well-versed in Regulatory publishing, is the key to success for life sciences organizations. At Freyr, we take pride in driving innovation through technology, and our Regulatory submission and publishing software, Freyr SUBMIT PRO. Got quick queries on eCTD submissions? Contact us today and pave the way for a seamless Regulatory future!
