Getting a product approved in the life sciences industry is no easy feat. It’s a highly regulated space where every submission goes through intense scrutiny. Along the way, Health Authorities (HAs) often come back with questions—requests for more data, clarifications, or additional documentation. And let’s be honest, these queries can be a real headache. If they’re not handled quickly and accurately, they can stall approvals, create compliance risks, and drive up costs.
Traditionally, managing these queries has been a manual, time-consuming process involving endless paperwork, email chains, and coordination across multiple teams. The result? Delays, inefficiencies, and plenty of frustration. But AI is changing the game. By automating key aspects of query management, AI-powered tools can help teams work faster and smarter. With capabilities like Natural Language Processing (NLP) and predictive analytics, AI is making it easier to interpret, prioritize, and respond to queries with greater accuracy—cutting down approval timelines and improving compliance.
In this blog, we’ll dive into how AI is transforming the way life sciences companies handle Health Authority queries and what it means for the future of regulatory operations.
Understanding Health Authority Queries
Health Authority queries are official requests for additional information or clarifications from regulatory agencies, such as the FDA, EMA, MHRA, and Health Canada, regarding a submitted dossier. These queries can pertain to clinical data, manufacturing processes, labeling, safety, and efficacy.
Common Challenges in Managing Health Authority Queries:
- High Volume of Queries: Regulatory authorities may request multiple clarifications, requiring quick and accurate responses.
- Stringent Timelines: Delays in query responses can lead to submission rejections or extended review timelines.
- Risk of Misinterpretation: Miscommunication can result in non-compliant responses, necessitating further queries and prolonging approvals.
- Manual Documentation Burden: Traditional query handling is document-intensive, leading to inefficiencies and human errors.
As regulatory requirements become more stringent, AI-driven solutions offer a way to streamline Health Authority query management, ensuring faster approvals and improved compliance.
How AI is Transforming Health Authority Query Management
Automation of Query Handling
AI-powered regulatory technology (RegTech) solutions can automate the end-to-end query management process. AI-driven platforms can:
- Categorize and prioritize queries based on urgency and complexity
- Route queries to the appropriate stakeholders within the regulatory team
- Automate query tracking and deadline management to prevent delays
Natural Language Processing (NLP) for Smart Query Interpretation
NLP-powered AI tools can analyze Health Authority queries, understand their context, and suggest relevant responses. These tools can:
- Extract key terms from queries to classify them into predefined categories
- Provide regulatory professionals with past responses to similar queries for reference
- Generate draft responses based on historical data and regulatory guidelines, reducing manual effort
AI-Driven Knowledge Management
AI enables centralized knowledge repositories where regulatory teams can access previously submitted responses, regulatory intelligence, and global submission guidelines. By leveraging machine learning, these repositories can:
- Identify patterns in Health Authority queries to anticipate future requests
- Ensure consistency in responses across different regulatory markets
- Minimize redundant work by suggesting pre-validated responses
Predictive Analytics for Proactive Query Resolution
AI can analyze past regulatory interactions and predict potential queries that may arise during submission review. This allows regulatory teams to:
- Preemptively address common Health Authority concerns in initial submissions
- Reduce back-and-forth communication with regulatory agencies
- Improve first-cycle approval rates by submitting more comprehensive dossiers
Workflow Optimization and Compliance Tracking
AI-powered workflow automation tools streamline query tracking, ensuring compliance with submission deadlines. These tools:
- Provide real-time dashboards for monitoring query status and progress
- Send automated alerts and reminders for pending responses
- Ensure adherence to region-specific regulatory timelines and guidelines
Benefits of AI-Driven Health Authority Query Management
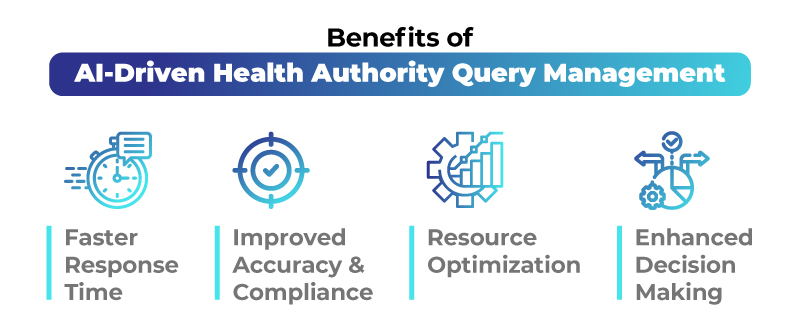
- Faster Response Time AI reduces turnaround time by automating query classification, retrieval of past responses, and response drafting. This leads to quicker query resolution and faster market approvals.
- Improved Accuracy and Compliance AI ensures that responses align with the latest regulatory guidelines, minimizing human errors and enhancing compliance.
- Resource Optimization By automating repetitive tasks, AI allows regulatory professionals to focus on strategic decision-making rather than manual query handling.
- Enhanced Decision-Making AI-driven insights enable regulatory teams to anticipate potential Health Authority concerns and proactively address them, reducing the risk of delays.
Real-World Applications & Industry Adoption
Many life sciences companies are already leveraging AI to enhance their regulatory operations. Some notable applications include:
- AI-powered chatbots assisting regulatory teams in drafting Health Authority query responses. One such example is Freya, an AI-powered chatbot for regulatory intelligence. Freya enables regulatory professionals to access real-time regulatory insights, automate data retrieval, and assist in crafting accurate responses to Health Authority queries. By leveraging Freya’s capabilities, teams can significantly reduce manual research efforts and enhance query response efficiency.
- Machine learning models analyzing historical Health Authority interactions to predict probable queries.
- Automated dossier review tools ensuring submission readiness before filing.
Conclusion
AI is revolutionizing how life sciences companies handle Health Authority queries, offering automation, predictive insights, and workflow optimization. By reducing response times, improving accuracy, and enhancing compliance, AI-driven solutions enable faster market approvals and improved regulatory efficiency. As the industry continues to embrace AI-powered regulatory technologies, companies that invest in digital transformation will gain a competitive edge.
One such innovative solution is Freyr Digital's upcoming Freya Fusion, an AI-enabled unified regulatory platform. Freya Fusion integrates AI and machine learning to automate workflows, provide real-time insights, and enhance collaboration across regulatory processes. With features like configurable workflows, timely notifications, and an AI-powered chatbot for interactive assistance, Freya Fusion is poised to set a new standard in Health Authority query management and overall regulatory affairs.
To stay ahead, regulatory teams should explore AI solutions like Freya Fusion that streamline Health Authority query management, minimize compliance risks, and drive faster approvals. The future of Health Authority query management is AI-powered, and the time to adopt these innovations is now.
