
The life science industry plays a significant role in maintaining public health and medical emergencies which is why there are thorough checks on it and strict compliances to follow. Regulatory compliances in the life science industry must be followed to the letter, a mistake on any part will lead to delayed and ineffective medicines.
Regulatory Documents are no easy tasks to handle, especially since all the documents, from research to procurement of the medicine, must be updated and kept for compliance checks. With the advent of computers and technology, Document Managment has slowly shifted from manual paper documents to electronic documents, which can be created, stored, reviewed, updated, and even collaborated on. Such a system is called an Electronic Regulatory Document Managment System.
Why shift to an Electronic Regulatory Document Management System?
With constant compliance checks and regular updates, managing life science regulatory documents can be cumbersome and error prone. Manual management of regulatory documents can lead to issues like:
- Version Control
Where outdated documents can be circulated. Working in siloed digital system or paper documents systems increases the chances of inefficiency in tracking, updating and correcting documents. This can lead to several documents being outdated or incomplete which can further cause confusion, mistakes, delays, and a compliance risk. - Accessibility Issue
Physical documents can be misplaced or lost, which can cause a huge loss of time and finances for companies. Siloed Document Management systems can face accessibility issues since it is worked on separately, making searching and updating documents time consuming. - Audit Challenges
Auditing requires quick access and view of the regulatory documents. Since manual documents can be misplaced or stored separately, they can be missed out during regulatory auditing and cause higher fines. - Compliance Risk
The overall risk with manual regulatory document management system is compliance error. Errors like Missed Deadlines, Inconsistent Documentation, Audit Trail issues and Slower Response time can increase compliance risk for many companies.
Benefits of an Electronic Regulatory Document Management Systems
An electronic regulatory document management system essentially functions as a digital hub where you can create, store, review, approve, distribute and archive all regulatory paperwork.
- Improved efficiency
Having a single repository of all regulatory documents will improve efficiency and functionality. Instead of multiple scattered documents that are potentially outdated or incorrect, the single repository can track and ensure the latest version of the document which eliminates version chaos and enhances workflow management. - Enhanced collaboration
An electronic document management system which is cloud hosted helps authorized users from multiple locations to work and efficiently collaborate on the document. With this, the reviewing and approval process of the documents can be sped up, increasing the turnaround time. - Secured and Simplified
Electronic System constantly strives to create stricter and tighter security measures to protect sensitive regulatory information. Along with regular security measures, electronic systems enable complete audit trails of all documents which simplify compliance audits. - Regulatory Compliance
Many rDMS have features that support regulatory compliance such as 21 CFR Part 11 for the FDA in the US. - Prepared for the future
With the advancement of AI, software solutions are now at the brink of a new era which can not only make regulatory document management error safe but also enhance the quality and speed of submissions.
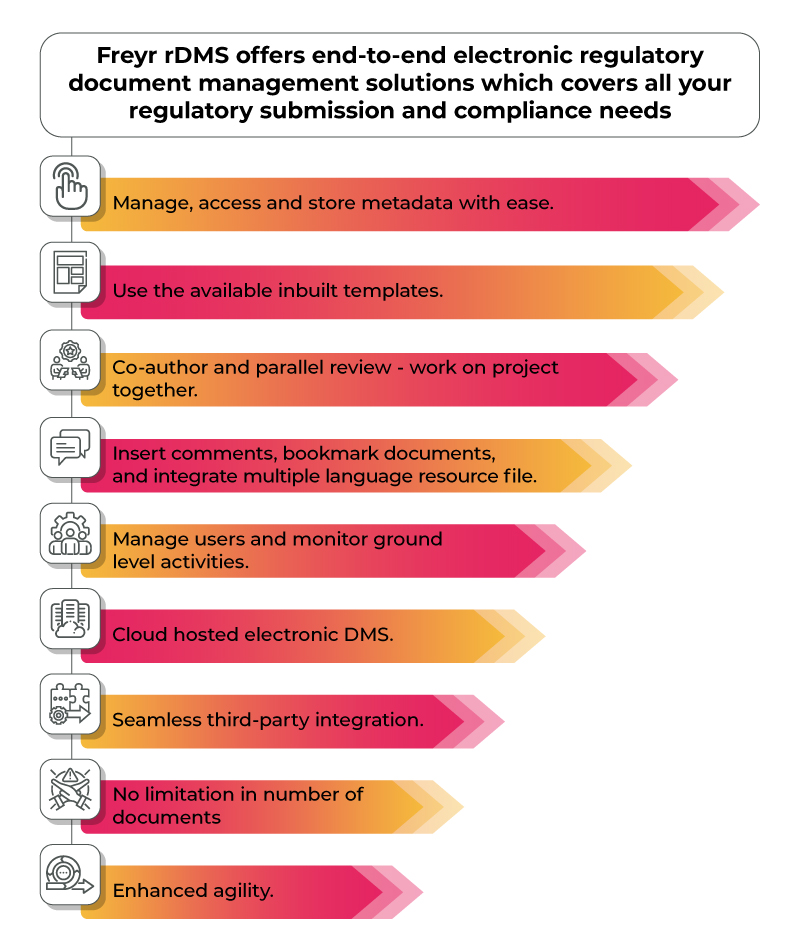
Choosing the right electronic regulatory document management systems is crucial especially when manually managing regulatory documents increases the risk of non-compliance with regulatory requirements. Further leading to fines and penalties, product delays or recalls and even reputational damage and loss of business opportunities. Freyr’s Electronic Document Management System Frery rDMS is a product of 10+ years of experience in providing regulatory solutions to 20+ countries around the world. Contact Us and make regulatory document management a breeze and minimize compliance risk.
